This is a Test…The Chemistry of your Pond Water
“Testing your Pond Water Could Save a Life”
Monitoring the water quality in your koi pond and performing regular tests of the pond water can give insight into what is going on inside the pond that you cannot see.
Clear water is not always how to tell if a pond is healthy. So, let’s dive in and talk about water quality testing. (pun intended)
Ammonia comes from fish waste, plant waste and other decomposing debris in the pond. It will burn a fish’s gills, the way smoke from a house fire will burn your lungs. This stops the ability of the fish to receive oxygen from the water.
- Ammonia begins to affect fish at 0.02ppm. At 0.25 ppm it starts to do damage to pond fish and long-term exposure can be fatal. Ammonia levels of 0.5 ppm and up are toxic. Toxicity of ammonia goes up exponentially as pH goes above 8.6.
- Ammonia is heavier than water. Therefore, it settles on the bottom of the pond.
- This is why your test sample needs to be taken from as deep as you can reach to be as accurate as possible.
- Ammonia is broken down by beneficial bacteria in the biological pond filter and turned into nitrites, then nitrates, which are less toxic to your fish.
- True aquatic plants such as hornwort, water hyacinths, water lettuce, cattails, arrow head, algae, etc. consume ammonia. Algae, in the early spring - before the bacteria has multiplied, may be a life saver to your fish.
- High readings of ammonia can be rendered less harmful with Pond Basics Ammonia Neutralizer and removed with Pond Basics Nitrifying Bacteria Plus.
pH expresses the acidity or alkalinity of the pond water. A reading of 7 is neutral, while a lower number is acidic and higher numbers are basic.
- A stabilized pH of a pond should be between 7.0 - 8.5.
- pH is a range, changing from sunrise to sunset.
- Limestone in a pond can raise the pH to 9 or more.
- Having a high pH will cause beneficial bacteria to go dormant, allowing string algae to take over, and may kill or stunt the growth of other pond plants.
- Hot sun shining down all day may make the pH levels raise. Thus, causing any trace amounts of ammonia to be 10 times as toxic.
Alkalinity is the water’s ability to resist changes in pH and a measure of the total concentration of bases including carbonates, bicarbonates, hydroxides and phosphates. The bases in the water will react with, and neutralize acids, which “buffers” changes in the pH.
- If alkalinity is low (below 80 ppm), a natural solution is to add pouches of crushed oyster shells into your waterfall. This releases calcium carbonate into the pond water and keeps the pH from swinging drastically. Think of it like taking an antacid to relieve heartburn.
Hardness is the naturally occurring calcium and magnesium present in the water. The more of these elements that are naturally in the water, the “harder” the water is.
- The harder the water is, the more resilient your koi and goldfish will be if herbicides from your lawn get into the water by accident.
Nitrites is the first product in the process of oxidizing harmful ammonia in the pond with beneficial bacteria.
- Although less readily toxic than ammonia, prolonged exposure to high levels of nitrites can weaken the ability for your fish’s gills to put oxygen into its blood.
- Nitrites start to become dangerous around 0.5 ppm and is toxic at 1 ppm.
Nitrates is the final stage in breaking down (oxidizing) harmful ammonia through the use of beneficial bacteria, such as Pond Basics Dry Starter Bacteria.
- Nitrates become toxic at 80 ppm.
- They are absorbed as nutrients, out of the water, through the roots of complex pond plants.
Phosphates are a naturally occurring form of phosphorus in pond water. It can become excessive through fish food, runoff, fertilizers, too much dead organic matter in the pond, etc.
- Excess amounts of phosphates in the water will contribute to cloudy, murky, green water.
- Over time, green water algae blooms can deplete pond water of oxygen.
- This is especially true in warmer temperatures and lead to harmful conditions for your pond fish.
- Phosphates can be removed from the water with aquatic pond plants, water treatments; like Pond Basics Natural Phosphate Binder and/or partial water changes.
Salt only needs to be tested if you are administering it to your pond as a preventative measure. Salt levels of 0.08-0.1 ppm will balance the water pressure inside and outside the fish’s body - ultimately causing less stress for your finned friends. * This can be achieved by dissolving approximately 1 cup of granulated salt per 100 gallons of pond volume into the pond water.
- Pond salt should be pure non-iodized salt, for example evaporated sea salt. Additives and anti-caking agents can cause chemical changes in the water that may result in drastic pH swings or an eco-system crash.
- Water temperatures will affect the solubility of salt in your pond. This means that in colder temperatures, salt may not dissolve into the pond water as well, and settle at the bottom of the pond. When the water warms up, the salt will dissolve again and could be too much.
- Take care to not over-salt your pond. Too much salt will cause an adverse effect and possibly kill your pond plants.
Water Temperature offers much explanation as to what is happening in your pond at certain times.
- Take note of your water temperature at every water testing to know how it is affecting the toxicity of ammonia levels, pH swings, dissolved oxygen levels, and so much more.
- Read our blog on “ The Importance of Your Pond Thermometer” for more details.
“The best defense is always a good offense.”
Regular testing of your pond water will put you in the know before anything visually goes wrong in your pond.
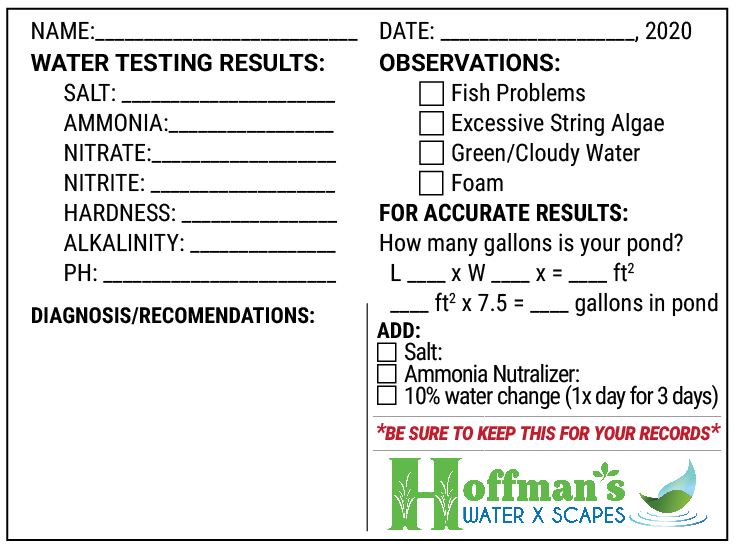
*Print the water test sheet (below) to keep record of your pond’s water quality. If a problem arises, bring this information in to the garden center for recommendations.